
Are we sterilising our instruments correctly?
At Mosman Village Dentistry we take infection control seriously and for that reason we maintain a high standard in sterilising our dental instruments.
It is an utmost importance to us that we can treat all our patients, the elderly, the young or the immunocompromised, with a confidence that there’s no chance of spreading any diseases by transmission of bacteria, viruses and other microbes. Many of our previous staff who have moved on to other career paths but still living near Mosman continue to return to us as patients. They say they feel safe coming to us due to the level of infection control measures they know and trust.
This confidence in prevention of infections is even more important during the pandemic of Covid-19. To find out more about our extra level of protection against Covid-19, please click here.
What is sterilisation of instruments?
Sterilisation is the process of killing all microorganisms on the surfaces and inside the chambers of any instruments we use in and around the mouth for dental procedures. By killing all the microorganisms we can prevent their growth and spreading any transmissible infectious diseases.
You may be aware that our practice is an accredited dental surgery by QIP.
This accreditation is a voluntary process to demonstrate our dedication to provide the highest standards of quality, safety and continuous improvement in our services. We have worked and have ongoing reviews with QIP, an independent third party who also work with private hospitals, to cross check and benchmark our standards in infection control procedures. We are put through rigorous tests and challenges to prove to ourselves that we are sterilising all our instruments to the highest standards every time and every day consistently.
So, how do we know if our instruments are clean and sterilised properly?
Firstly, we use single-use instruments whenever possible. Also, many surfaces are covered with single-use barrier adhesives or disposable barrier plastic sleeves. Sorry, it’s not very environmentally friendly, we go through a fair bit of plastic. As an example, on average, we use 20 pairs of gloves between a dentist and a nurse to see one patient. This is the standard precaution, preventing infectious diseases is our priority before minimizing plastics.

Secondly, every time we put our instruments through the steriliser, each item is cleaned and processed first and then packaged with indicators that later confirm sterilisation has been successful.
Thirdly, our steriliser has built in quality measures of temperature, duration of sterilisation water cleanliness etc. and it either passes or fails each sterilisation cycle. For example, if the door of the steriliser was not sealed off properly, there will be a warning and we would have to start the sterilising cycle all over again
And lastly, and this is just in case the steriliser is not reporting back to us accurately, we also validate the steriliser itself and test its function with biologic tests. This biological indicator test is done on regular basis to ensure there’s no mechanical fault or operator errors causing any microorganisms to survive.
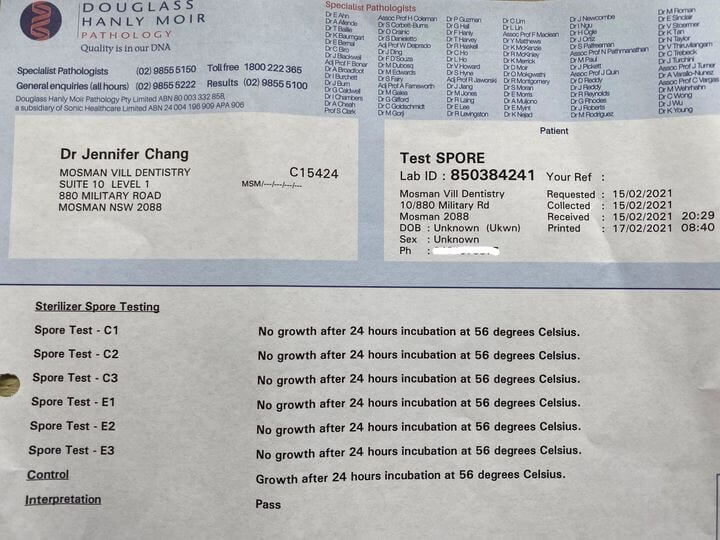
Here’s our latest test results and I am pleased to say we passed with flying colours.
What are biological tests?
Biological indicators (also known as spore tests) are the most reliable method to monitor sterilisation process by directly killing known highly resistant microorganisms.
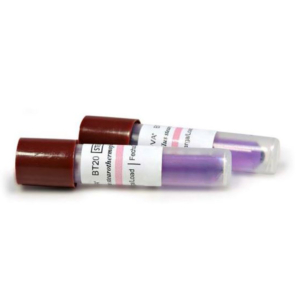
Inside each biologic indicator is microorganisms such as Geobacillus Stearothermophilus spores and we used 7 indicators on this occasion.
The indicators are marked C1, C2, C3, E1, E2, E3 and Z.
E1 and C1 are used in the first validation cycle. E1 is placed in the centre of the most dense and crowded pack of instruments in the centre of the sterilisation chamber. C1 is placed in the most “cold spot” of the chamber.
E2 and C2 are placed in exactly the same location and same circumstance with the same but cooled down instruments.
After the second cycle has completed and cooled the process is repeated again for the third and final cycle using E3 and C3.
The above 3 cycles are run consecutively as the aim of validation is to prove consistency of sterilization process.
Once the above 6 indicators have been through the sterilization process, we add the 7th control indicator labelled Z that has not been through sterilization.
The 7 indicators are sent to a Pathology Laboratory for incubation for 24 hours and observed up to 48th hour for any growth of microorganisms.
Interpreting the results and conclusion
No growth in all 6 indicators that have been through the sterilisation process proves all microorganisms have been killed. Growth in the control Z indicator that did not go through sterilisation proves the indicators contain microorganisms.
This steriliser validation with biologic tests take hours as the steriliser has to run each cycle and cool down between the cycles. We are committed to adhering to this protocol to ensure safety to our patients and our staff.
Last updated by Dr Jennifer Chang
5/8/2021





